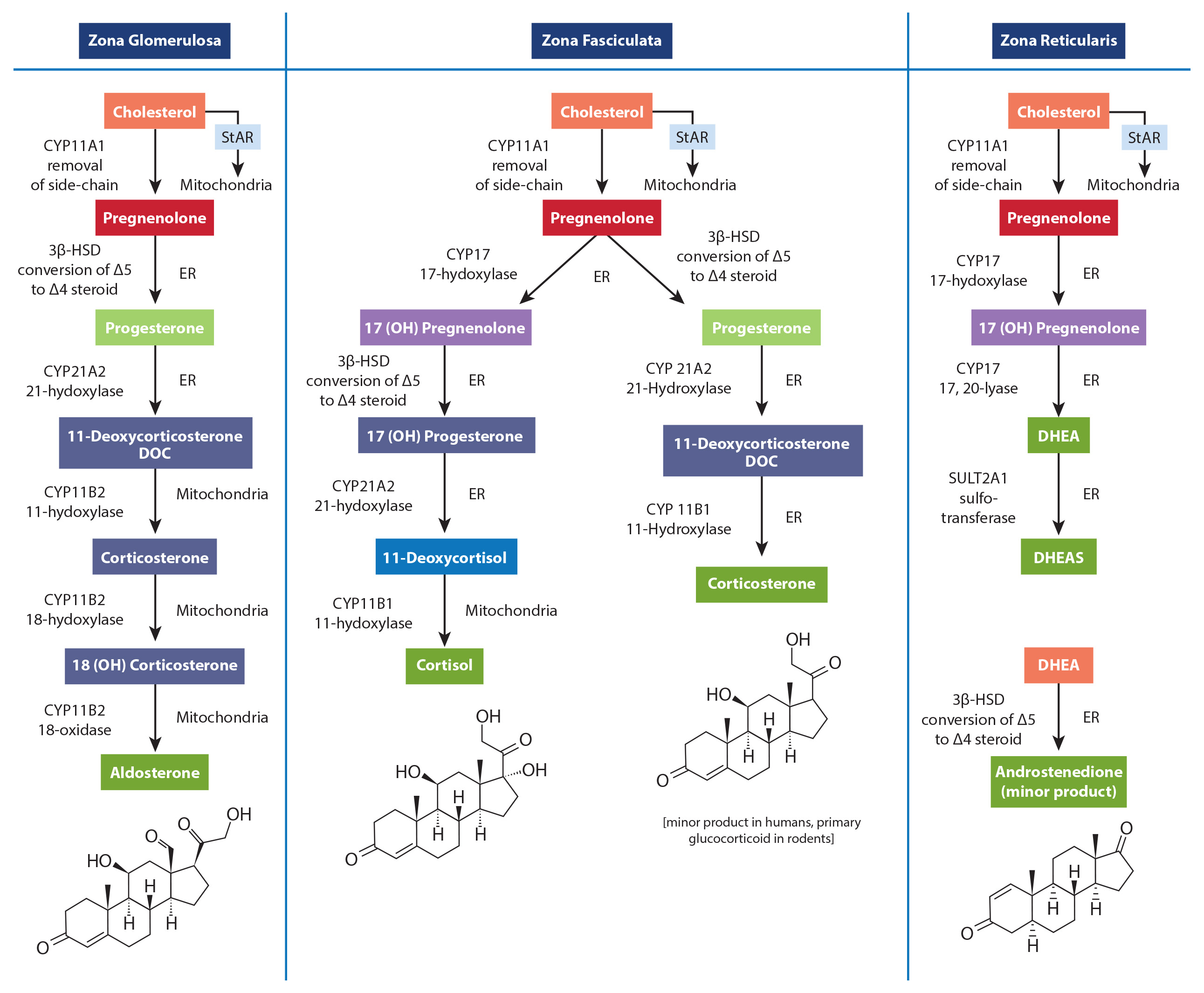
Steroid horomones produced by the adrenal cortex are potent molecules regulating important human physiology, including blood sugar levels, inflammation, blood pressure and volume. The frequency of pathophysiological adrenal gland disorders in humans spans a wide range depending on the particular disorder. Hyperaldasteronism occurs in up to 10% of hypertensive individuals; while Cushing's Syndrome occurs in 1/100,000 people, though women are 5x more likely to be affected than men.
The goal of this research is to understand the RNA regulatory mechanisms controlling the metabolism of steroid hormone produced in the adrenal cortex. Our experiments are designed to quantify RNA metabolism, specifically, the kinetics of indvidual steps of RNA metabolism in human H295R cells during a timecourse response to a steroidogenic stimulus producing cortisol and aldosterone. The quantification relies on a combination of biochemical methods coupled to RNA-sequencing followed by principled computational analysis. We will use metabolic labeling to infer the kinetics of synthesis, processing, and degradation. Ribosome profiling will be utilized to assess translation. Integration of these data will result in the 1) discovery and quantification of RNA regulatory events and 2) inference of putative regulators (RBPs, lncRNAs, and miRNAs). Finally, we will perturb specific putative regulators and regulatory events to determine their impact on steroidogenesis.
- Generate human steroidogenic transcriptome.
- Infer synthesis, processing, and degradation rates.
- Identify and quantify translation of ORFs.
- Integrative analysis
- Identify regulatory events.
- Infer putative regulators.
- Focus on ZFP36 family and/or miRNAs.
Extra focus will be given to genes with established roles in steroidogenesis (signal transducers, transcriptional regulators, and biosynthetic enzymes). Genes with behavior mimmicking the known important genes may represent novel regulators of steroidogenesis.
Need to include a table describing the data:
6 timepoints (including untreated) in replicate.
- STAR 2-pass mapping strategy
- Map each sample using GENCODEv25
- Concatenate all new junctions (some filtering).
- Re-map all sample including new junctions.
- Run stringtie with default parameters on each sample
- Merge stringtie-generated GTF using the following samples:
- All samples
- Total RNA samples only
- 4sU samples only
- Assess utility of new transcripts.
- Probably filter out "intronic sense" transcripts since these could interfere with quantification of both primary and mature RNA levels.
- Many divergent transcripts that will not interfere with quantification and are likely to be filtered out due to low expression in later steps in the analysis.